Developer Offer
Try ImaginePro API with 50 Free Credits
Build and ship AI-powered visuals with Midjourney, Flux, and more — free credits refresh every month.
AI Enhances Cancer Prediction From Pathology Images
The Challenge in Predicting Immunotherapy Success
Recent breakthroughs in immunotherapy, especially with drugs like pembrolizumab, have offered new hope for patients with metastatic colorectal cancer (CRC) and triple-negative breast cancer (TNBC). The effectiveness of these treatments hinges on accurately identifying specific biomarkers in patients, such as microsatellite instability (MSI) and programmed death-ligand 1 (PD-L1).
However, traditional methods for detecting these biomarkers—like immunohistochemistry (IHC) and next-generation sequencing (NGS)—have significant drawbacks. They are often labor-intensive, time-consuming, and rely on subjective interpretation by highly trained pathologists, which can lead to inconsistent results. These challenges create a critical need for faster, more reliable, and objective tools to guide clinical decisions.
Introducing DuoHistoNet A Dual-Modality AI Framework
To address these limitations, researchers have developed an innovative artificial intelligence (AI) framework called DuoHistoNet. This efficient, transformer-based model takes a unique dual-modality approach by synergistically analyzing two different types of pathology images from the same patient:
- Hematoxylin & Eosin (H&E) images: The standard, cost-effective stain used in pathology to see tissue morphology.
- Immunohistochemistry (IHC) images: A specific stain used to highlight the presence of key proteins, like the biomarkers in question.
By integrating information from both H&E and IHC images, the model hypothesizes it can achieve a more accurate and comprehensive understanding of the tumor's characteristics than by analyzing either image type alone. This framework is designed to be flexible, allowing it to work with H&E images, IHC images, or both, and its predictive thresholds can be customized for different clinical needs.
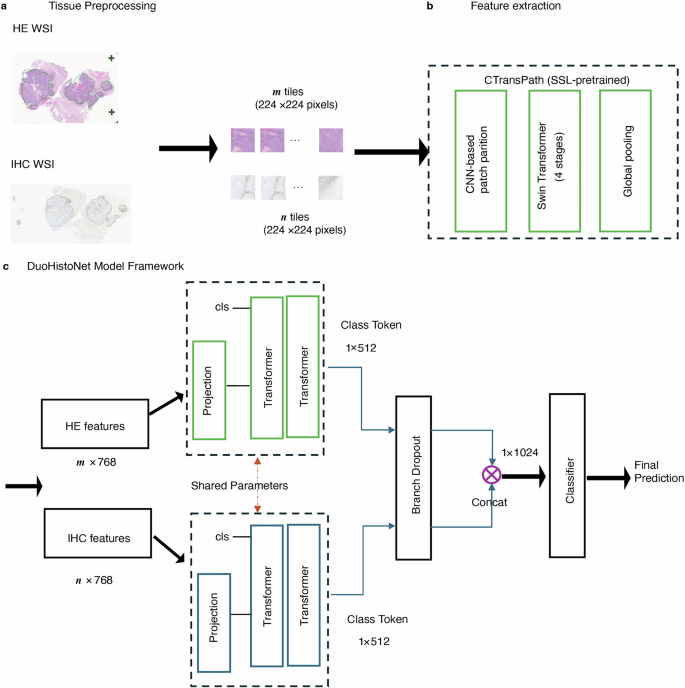
How the AI Model Works Under the Hood
The DuoHistoNet framework operates on a sophisticated three-stage pipeline. First, whole slide images (WSIs) are digitized and pre-processed, where tissue is automatically segmented and broken down into smaller, analyzable tiles.
Next, a powerful feature extractor, pre-trained on a massive dataset of histopathology images, analyzes each tile to generate a detailed feature vector. This model combines the strengths of convolutional neural networks (CNNs) for local feature detection with Swin Transformers for understanding global context.
Finally, an aggregation module uses a transformer-based architecture to process all the tile features from a slide. For the dual-modality approach, the model uses two parallel branches—one for H&E features and one for IHC features. The outputs are then combined to produce a final, highly accurate WSI-level prediction for the biomarker status.
Achieving Clinical-Grade Accuracy in Biomarker Prediction
The performance of DuoHistoNet was rigorously evaluated on large patient cohorts, including over 20,000 CRC cases and 15,000 breast cancer cases. The results demonstrated exceptional, clinical-grade accuracy.
For predicting MSI/MMRd status in colorectal cancer, the dual-modality model achieved an area under the receiver operating characteristic (AUROC) score exceeding 0.97. This was a significant improvement over models that used only H&E images (0.92 AUROC) or only IHC images (0.95 AUROC).
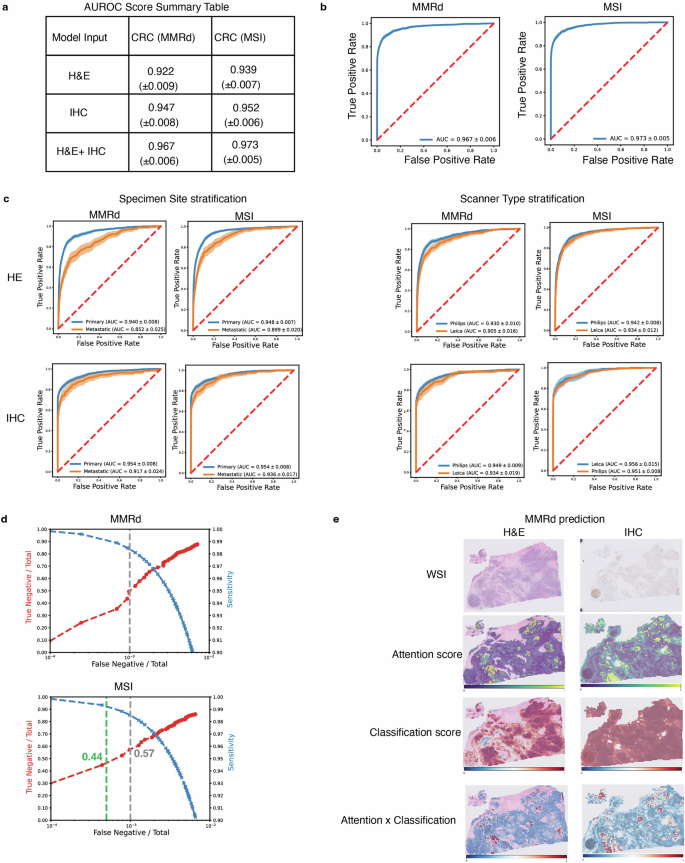
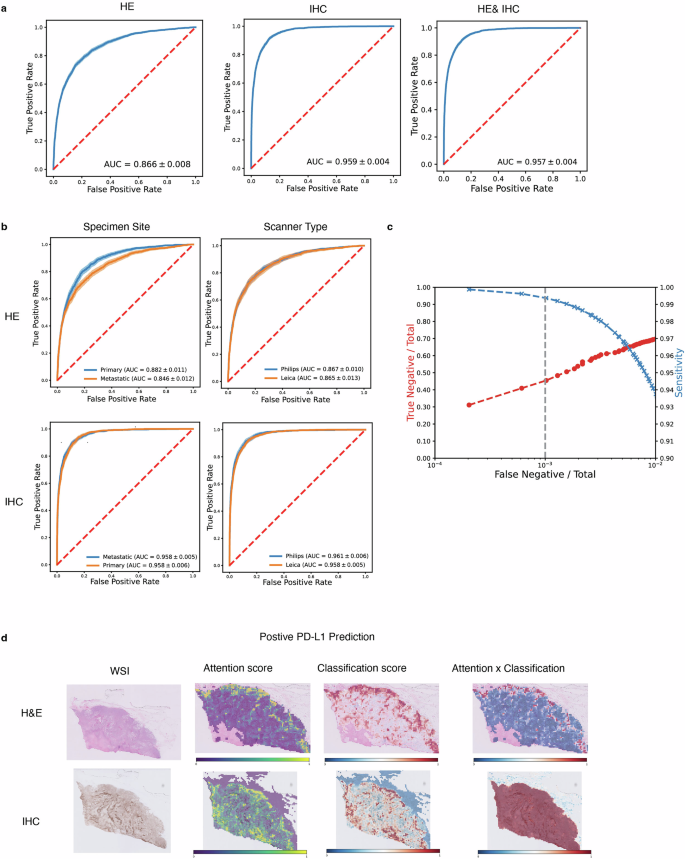
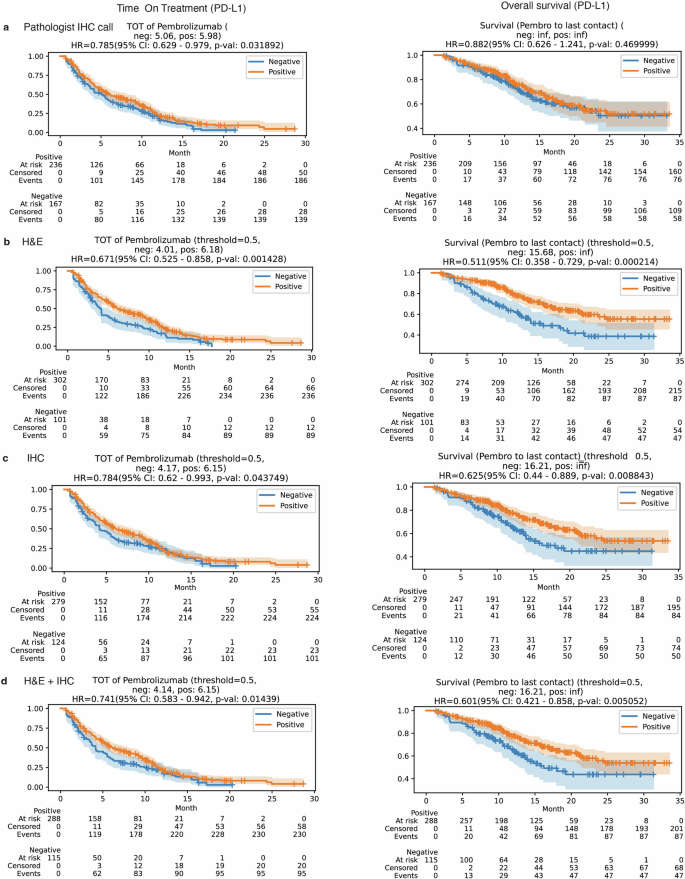
Similarly, for predicting PD-L1 status in breast cancer, the IHC-only and dual-modality models both achieved a high AUROC of approximately 0.96, far surpassing the H&E-only model (0.87 AUROC). This highlights that while H&E provides useful context, the specific IHC stain is a powerful predictor for PD-L1. The model also proved robust, performing consistently across different image scanners and tissue sites (primary vs. metastatic).
AI Predictions Link Directly to Patient Survival
Beyond just predicting biomarkers, the study investigated whether the AI's predictions had real-world clinical relevance. By analyzing time-on-treatment and overall survival data for patients receiving pembrolizumab, researchers found a strong correlation.
Patients with CRC whom the AI predicted to be biomarker-positive (MSI-H/MMRd) showed significantly longer survival, closely mirroring the outcomes of patients identified through traditional testing. The dual-modality model's predictions showed the strongest association with improved clinical outcomes.
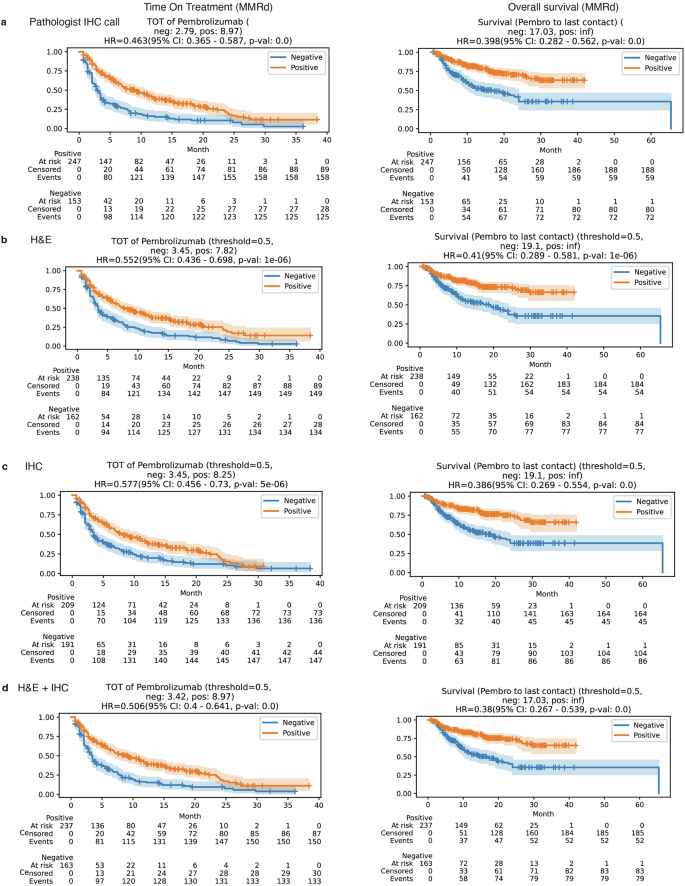
Most strikingly, for breast cancer patients, the PD-L1 status predicted by the AI model (particularly the H&E-only model) was a superior predictor of survival outcomes than the PD-L1 scores manually determined by pathologists. This groundbreaking finding suggests that AI may capture subtle morphological features invisible to the human eye that are more indicative of treatment response, potentially leading to a reevaluation of how patients are selected for immunotherapy.
The Future of AI in Personalized Oncology
This study demonstrates the immense potential of integrating advanced AI tools into clinical pathology. The DuoHistoNet model not only enhances predictive accuracy but also provides a more precise prognosis than current biomarker assessments. Its ability to function as a highly effective pre-screening tool could significantly reduce the workload in pathology labs, cut costs, and expedite patient access to critical treatments.
By generating heatmaps to visualize which tissue regions most influence its predictions, the model also serves as an interpretive aid for pathologists, potentially uncovering novel diagnostic patterns. Future work aims to expand this framework into a fully multimodal system, incorporating genomic data and additional IHC stains to further refine cancer subtyping and treatment selection.
Ultimately, the clinical adoption of tools like DuoHistoNet promises to improve the precision and efficiency of cancer patient evaluation, heralding a new era of AI-driven personalized medicine.
Compare Plans & Pricing
Find the plan that matches your workload and unlock full access to ImaginePro.
| Plan | Price | Highlights |
|---|---|---|
| Standard | $8 / month |
|
| Premium | $20 / month |
|
Need custom terms? Talk to us to tailor credits, rate limits, or deployment options.
View All Pricing Details

